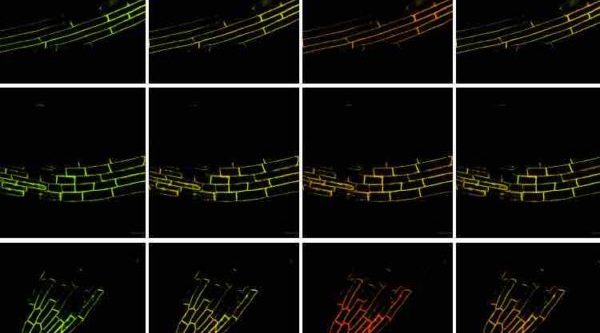
A team of Clemson University scientists has achieved a breakthrough in the genetics of senescence in cereal crops with the potential to dramatically impact the future of food security in the era of climate change.
The collaborative research, which explores the genetic architecture of the little understood process of senescence in maize (a.k.a. corn) and other cereal crops, was published in The Plant Cell, one of the top peer-reviewed scientific journals of plant sciences. Rajan Sekhon, a plant geneticist and an assistant professor in the College of Science’s department of genetics and biochemistry, is the lead and corresponding author of the paper titled “Integrated Genome-Scale Analysis Identifies Novel Genes and Networks Underlying Senescence in Maize.”
“Senescence means ‘death of a cell or an organ in the hands of the very organisms it is a part of,’ ” Sekhon said. “It happens pretty much everywhere, even in animals. We kill the cells we don’t need. When the weather changes in fall, we have those nice fall colors in trees. At the onset of fall, when the plants realize that they cannot sustain the leaves, they kill their leaves. It is all about the economy of energy.”
As a result, the leaves die off after their show of color. The energy scavenged from the leaves is stored in the trunk or roots of the plant and used to quickly reproduce leaves next spring. This makes perfect sense for trees. But the story is quite different for some other edible plants, specifically cereal crops like maize, rice and wheat.
“These crops are tended very carefully and supplied excess nutrients in the form of fertilizers by the farmers,” Sekhon said. “Instead of dying prematurely, the leaves can keep on making food via photosynthesis. Understanding the triggers for senescence in crops like maize means scientists can alter the plant in a way that can benefit a hungry world.”
Sekhon, whose research career spans molecular genetics, genomics, epigenetics and plant breeding, established his lab in 2014 as an assistant professor. He has played a key role in the development of a “gene atlas” widely used by the maize research community. He has published several papers in top peer-reviewed journals investigating the regulation of complex plant traits.
“If we can slow senescence down, this can allow the plant to stay green – or not senesce – for a longer period of time,” Sekhon said. “Plant breeders have been selecting for plants that senesce late without fully understanding how senescence works at the molecular level.”
These plants, called “stay-green,” live up to their name. They stay green longer, produce greater yields and are more resilient in the face of environmental factors that stress plants, including drought and heat.
But even with the existence of stay-green plants, there has been little understanding about the molecular, physiological and biochemical underpinnings of senescence. Senescence is a complex trait affected by several internal and external factors and regulated by a number of genes working together. Therefore, off-the-shelf genetic approaches are not effective in fully unraveling this enigmatic process. The breakthrough by Sekhon and his colleagues was the result of a systems genetics approach.
Sekhon and the other researchers studied natural genetic variation for the stay-green trait in maize. The process involved growing 400 different maize types, each genetically distinct from each other based on the DNA fingerprint (i.e., genotype), and then measuring their senescence (i.e., phenotype). The team then associated the “genotype” of each inbred line with its “phenotype” to identify 64 candidate genes that could be orchestrating senescence.
“The other part of the experiment was to take a stay-green plant and a non-stay-green plant and look at the expression of about 40,000 genes during senescence,” Sekhon said. “Our researchers looked at samples every few days and asked which genes were gaining expression during the particular time period. This identified over 600 genes that appear to determine whether a plant will be stay-green or not.
“One of the big issues with each of these approaches is the occurrence of false positives, which means some of the detected genes are flukes, and instances of false negatives, which means that we miss out on some of the causal genes.”
Therefore, Sekhon and his colleagues had to painstakingly combine the results from the two large experiments using a “steams genetics” approach to identify some high-confidence target genes that can be further tested to confirm their role in senescence. They combined datasets to narrow the field to 14 candidate genes and, ultimately, examined two genes in detail.
“One of the most remarkable discoveries was that sugars appear to dictate senescence,” Sekhon said. “When the sugars are not moved away from the leaves where these are being made via photosynthesis, these sugar molecules start sending signals to initiate senescence.”
However, not all forms of sugar found in the plants are capable of signaling. One of the genes that Sekhon and colleagues discovered in the study appears to break complex sugars in the leaf cells into smaller sugar molecules – six-carbon sugars like glucose and fructose – that are capable of relaying the senescence signals.
“This is a double whammy,” Sekhon said. “We are not only losing these extra sugars made by plants that can feed more hungry mouths. These unused sugars in the leaves start senescence and stop the sugars synthesis process all together.”
The implications are enormous for food security. The sugars made by these plants should be diverted to various plant organs that can be used for food.
“We found that the plant is carefully monitoring the filling of the seeds. That partitioning of sugar is a key factor in senescence. What we found is there is a lot of genetic variation even in the maize cultivars that are grown in the U.S.”
Some plants fill seeds and then can start filling other parts of the plant.
“At least some of the stay-green plants are able to do this by storing extra energy in the stems,” Sekhon said. “When the seed is harvested, whatever is left in the field is called stover.”
Stover can be used as animal feed or as a source of biofuels. With food and energy demand increasing, there is a growing interest in developing dual-purpose crops which provide both grain and stover. As farmland becomes scarce, plants that senesce later rise in importance because they produce more overall energy per plant.
The genes identified in this study are likely performing the same function in other cereal crops, such as rice, wheat and sorghum. Sekhon said that the next step is to examine the function of these genes using mutants and transgenics.
“The ultimate goal is to help the planet and feed the growing world. With ever-worsening climate, shrinking land and water, and increasing population, food security is the major challenge faced by mankind,” Sekhon said.
In addition to Sekhon, other contributors include Rohit Kumar, Christopher Saski, Arlyn Ackerman, William Bridges, Barry Flinn and Feng Luo of Clemson University; Timothy Beissinger of the University of Gottingen; and Matthew Breitzman, Natalia de Leon and Shawn Kaeppler of the University of Wisconsin-Madison.
Read the paper: The Plant Cell
Article source: Clemson University
Image: Clemson University/College of Science