




Understanding the role of a key protein in plant immunity could inform the development of crops that are resistant to multiple pathogens.
Up to 30 per cent of crop yields worldwide each year are lost to pathogenic infection. Understanding how to make plants more resilient to infection is vital for future food security. Now researchers have uncovered the critical role of a linker histone protein, called H1, during plant immune responses to bacterial and fungal infections.
“Previous studies on Arabidopsis plants revealed that H1 is important for healthy growth and development,” says Arsheed Sheikh, who worked on the project with Heribert Hirt and co-workers. “Linker histones are known to regulate infection in animals, but their role in plant infection and immunity has never been explored.”
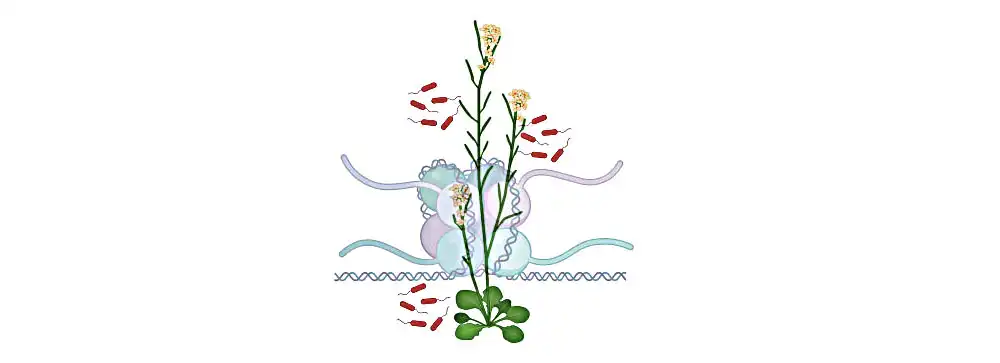
In animal and plant cells, fundamental units called nucleosomes contain DNA wrapped around a protein complex, and are critical for regulating genetic information. The individual nucleosomes are connected by linker DNA. Linker histone H1 holds the exit/entry site of linker DNA like a clip, thus regulating the unwinding and flexibility of nucleosomes.
“In plants like Arabidopsis, we find three isoforms of H1,” says Sheikh. “Normally, H1 suppresses gene expression — this includes the defense genes of the immune system.”
The team explored mutant Arabidopsis plants with all three H1 isoforms knocked out. They grew wild-type and mutant plants under controlled conditions and then infected them with either the bacterial pathogen Pseudomonas syringae or the fungal pathogen Botrytis cinerea. After three days, they compared the severity of infection between the different groups of plants.
“The mutant plants were resistant to both bacterial and fungal infection when compared to wild-type plants,” says Sheikh. “The knockout mutant had higher levels of defense gene expression and the immune response hormone salicylic acid.”
Probing H1’s role further, however, the team were surprised to find that the mutant plants lacked defense-priming ability. In other words, when subjected to a small dose of a pathogen some time after initial infection, the plants showed no enhanced immune response. Like vaccination, priming a plant with a small pathogen dose can boost its immunity. The lack of defense priming in the mutant plants suggests H1 plays a critical role in priming.
“This fundamental knowledge could help generate smart crops that are resistant to multiple infectious agents simultaneously,” says Hirt. “However, this study also serves as a cautionary warning that it is important to study both the direct and indirect effects of a given mutation in genetically modified plants.”
Read the paper: Nucleic Acid Research
Article source: KAUST
Image: Illustration of the model plant Arabidopsis. The genetic material is present as chromatin, which consists of DNA wrapped around a histone protein complex in cells. The linker histone H1 modulates the plant’s immunity against pathogens. Credit 2023 KAUST; Arsheed Sheikh.



Coating crop seeds with bacteria found on a desert shrub boosts yields in hot fields.
Bacteria plucked from a desert plant could help crops survive heatwaves and protect the future of food.
Global warming has increased the number of severe heatwaves that wreak havoc on agriculture, reduce crop yields and threaten food supplies. However, not all plants perish in extreme heat. Some have natural heat tolerance, while others acquire heat tolerance after previous exposure to higher temperatures than normal, similar to how vaccines trigger the immune system with a tiny dose of virus.
But breeding heat tolerant crops is laborious and expensive, and slightly warming entire fields is even trickier.
There is growing interest in harnessing microbes to protect plants, and biologists have shown that root-dwelling bacteria can help their herbaceous hosts survive extreme conditions, such as drought, excessive salt or heat.
“Beneficial bacteria could become one of the quickest, cheapest and greenest ways to help achieve sustainable agriculture,” says postdoc Kirti Shekhawat. “However, no long-term studies have proven they work in the real world, and we haven’t yet uncovered what’s happening on a molecular level,” she adds.
To fill this knowledge gap, Shekhawat, along with a team led by Heribert Hirt, selected the beneficial bacteria SA187 that lives in the root of a robust desert shrub, Indigofera argentea. They coated wheat seeds with the bacteria and then planted them in the lab along with some untreated seeds. After six days, they heated the crops at 44 degrees Celsius for two hours. “Any longer would kill them all,” says Shekhawat.
The untreated wheat suffered leaf damage and ceased to grow, while the treated wheat emerged unscathed and flourished, suggesting that the bacteria had triggered heat tolerance. “The bacteria enter the plant as soon as the seeds germinate, and they live happily in symbiosis for the plant’s entire life,” explains Shekhawat.
The researchers then grew their wheat for several years in natural fields in Dubai, where temperatures can reach 45 degrees Celsius. Here, wheat is usually grown only in winter, but the bacteria-bolstered crops consistently had yields between 20 and 50 percent higher than normal. “We were incredibly happy to see that a single bacterial species could protect crops like this,” says Shekhawat.
The team then used the model plant Arabidopsis to screen all the plant genes expressed under heat stress, both with and without the bacteria. They found that the bacteria produce metabolites that are converted into the plant hormone ethylene, which primes the plant’s heat-resistance genes for action. “Essentially, the bacteria teach the plant how to use its own defense system,” says Shekhawat.
Thousands of other bacteria have the power to protect plants against diverse threats, from droughts to fungi, and the team is already testing some on other crops, including vegetables. “We have just scratched the surface of this hidden world of soil that we once dismissed as dead matter,” says Hirt. “Beneficial bacteria could help transform an unsustainable agricultural system into a truly ecological one.”
Read the paper: EMBO reports
Article source: KAUST
Image: Studies have shown that root-dwelling bacteria can help plants and crops survive extreme conditions, such as drought, excessive salt or heat. Credit: Anastasia Serin, KAUST



Use of saline water to irrigate crops would bolster food security for many arid countries; however, this has not been possible due to the detrimental effects of salt on plants. Now, researchers at KAUST, along with scientists in Egypt, have shown that saline irrigation of tomato is possible with the help of a beneficial desert root fungus. This represents a new key technology for countries lacking water resources.
“Salt in irrigation water is one of the most significant abiotic stresses in arid and semiarid farming,” says former KAUST postdoc Mohamed Abdelaziz, who worked on the project team alongside Heribert Hirt. “Improving plant salt tolerance and increasing the yield and quality of crops is vital, but we must achieve this in a sustainable, inexpensive way.”
The root fungus Piriformospora indica forms beneficial symbiotic relationships with many plant species, and previous research indicates it boosts plant growth under salt stress conditions in barley and rice. While initial studies suggest the fungus can improve growth in tomato plants under long-term saline irrigation, the mechanisms behind the process are unclear. Also, little is known about the fungal-plant interaction throughout the entire growing season.
“Plant salt tolerance is a complex trait influenced by many factors,” says Abdelaziz. “The salt-tolerance mechanism depends on the correct activation of salt tolerance genes, stresses on cell membranes and the buildup of toxic sodium ions. We monitored growth performance over four months in tomato plants colonized with P. indica and in an untreated control group, both grown commercial style in greenhouses. We examined genetic and enzymatic responses to salt stress in both groups.”
The main threat to plants under salt stress is the buildup of sodium ions, which affects plant metabolism, and leaf and fruit growth. For example, excessive sodium in shoots and roots disrupts levels of potassium, which is vital for multiple growth processes from germination to enzyme activation.
The team showed that colonization by P. indica increased the expression of a gene in leaves called LeNHX1, one of a family of genes responsible for removing sodium from cells. Furthermore, potassium levels in leaves, shoots and roots of the P. indica group were higher than in controls. P. indica also increased levels of antioxidant enzyme activity, offering further protection.
“Colonization with P. indica boosted tomato fruit yield by 22 percent under normal conditions and 65 percent under saline conditions,” says Abdelaziz. “Colonizing vegetables provides a simple, low-cost method suitable for all producers, from smallholders to large-scale farming.”
Read the paper: Scientia Horticulturae
Article source: KAUST
Image credit: Capri23auto / Pixabay