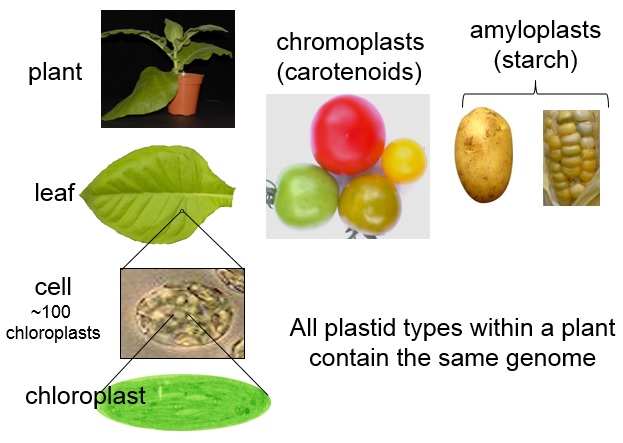
Plants in Action!
Alert: Teachers of plant physiology!
Have you ever wanted a free, on-line textbook written by experts and regularly updated?
Plants in Action is an on-line resource for students and academics teaching plant function to undergrads, published by the Australian and New Zealand societies of plant science. Each chapter has up to 100 illustrations suitable for Powerpoint presentations. It is also ideal for graduate students and post-docs in molecular biology looking for the whole-plant context for their work. Of the original 20 chapters, ten have been fully revised.
Here, we speak to Professor Susanne Schmidt (The University of Queensland) and Chief Editor Professor Rana Munns (CSIRO) about their work in developing this fantastic resource.
Could you begin by describing the Plants in Action (PiA) textbook and how the idea first came about?
The original editors and contributors produced a textbook on plant function that used examples from the southern hemisphere, with view of adaptations in nature to performance in cultivation. They were motived to communicate the strong plant science in Australia and New Zealand. PiA was born as a textbook in 1999, and ten years later went open-access and free online.
Who are your target audience?
Undergraduate students, educators, practitioners and researchers, and others interested in plants and how they function. PiA gets thousands of hits per day from around the globe, including developing countries.
What topics do you cover?
To the best of my knowledge, PiA is the only comprehensive plant science textbook with a southern hemisphere perspective. It covers molecular, cellular, and whole-plant function, in ecophysiology and vegetation-environment interactions, from Antarctica to the tropics. PiA features plants that are some of the best studied genetic models and crops, as well as wild plants.
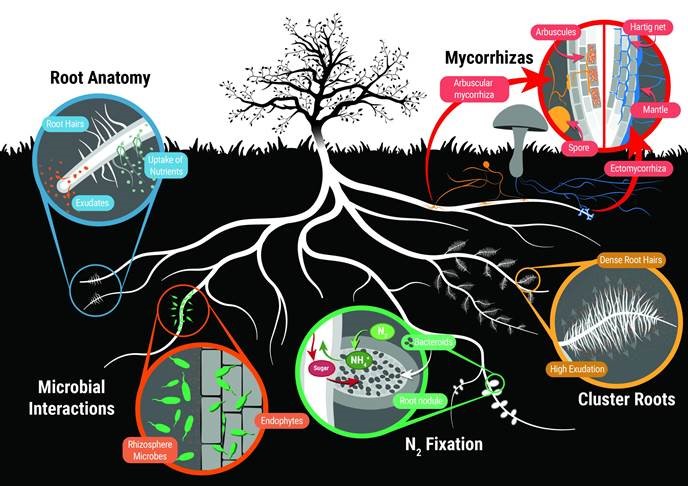
The topics covered in Chapter 4: Nutrient uptake by plants involving beneficial microorganisms. Image credit: Scott Buckley, The University of Queensland.
Who has contributed to the textbook, and how did you enlist potential collaborators?
PiA was written by Australian and New Zealand plant scientists from a range of institutions, many of whom have worked on both editions. Chief Editor Dr Rana Munns and the chapter editors find new contributors if the original authors are not available, although occasionally authors volunteer contributions
What changes have you made in the second edition, and how are the revisions coming along?
PiA2 is being updated to reflect recent advances in plant science, and has a new look as software enables ever more attractive layouts, with no limit for images and illustrations. PiA can be read online and is easily printed, which is important for internet-challenged regions and for students wanting to add notes. Ten chapters are fully updated with several chapters expanded.
Revisions can be made instantly (as a wiki) but take a little longer if the expert skills of our IT assistants are required. Complete revisions of chapters are slower to come on board.
Encouragingly, some excellent contributions have recently been made by junior scientists who see a strong value in developing an open-access resource to share their expertise widely.
You mentioned the text can be translated into different languages. How might users go about getting a textbook in their native language?
They can contact the Australian Society of Plant Scientists (admin@asps.org.au) and receive Word or PDF versions of individual chapters, with permission to translate and reproduce.
The project is sponsored by the Australian and New Zealand societies of plants scientists, ACIAR, and the University of Queensland. Could you elaborate on how this funding was acquired, if possible?
ACIAR (Australian Centre for International Agricultural Research) was our first sponsor. They saw the value of an open-access resource, particularly for plant biologists in developing countries and the free exchange of knowledge. The University of Queensland (UQ) came on board to provide the host server as PiA supports teaching at UQ with strengths in agriculture and environmental science. Sponsorship was also provided by the Western Sydney University, the University of Western Australia, and the Australian Centre for Plant Functional Genomics. The societies of plant science in Australia and New Zealand are ongoing supporters and PiA remains predominantly the product of their members.

Are you looking to expand on the work in the future? How can potential contributors get involved?
It would be marvelous to have a person with time and expertise to develop further materials to add to PiA2 in collaboration with editors, authors, educational designers, and students. This could include material specifically designed for schools and interactive learning tools for students of all levels. We welcome all contributors (irrespective of their connections to the plant societies), and they can contact Rana or any chapter editor.
Without plants, Earth would not give us habitat, food and materials. But with 25% of the global flora threatened with extinction, we need more people to understand plants, and what we need to do protect them and their habitats.

The first edition of Plants in Action was published by the Australian and New Zealand societies of plant science as a hardcover book in 1999, which is also now free on-line (http://plantsinaction.science.uq.edu.au/edition1/).
The PiA text can be translated into other languages. Please direct enquiries to the Chief Editor Professor Rana Munns via the Australian Society of Plant Scientists: admin@asps.org.au.
All images are courtesy of the Plants in Action team, and are used with permission.