At July’s New Breeding Technologies workshop held in Gothenburg, Sweden, Dr. Staffan Eklöf, Swedish Board of Agriculture, gave us an insight into their analysis of European Union (EU) regulations, which led to their interpretation that some gene-edited plants are not regulated as genetically modified organisms. We speak to him here on the blog to share the story with you.
Could you begin with a brief explanation of your job, and the role of the Competent Authority for GM Plants / Swedish Board of Agriculture?
I am an administrative officer at the Swedish Board of Agriculture (SBA). The SBA is the Swedish Competent authority for most GM plants and ensures that EU regulations and national laws regarding these plants are followed. This includes issuing permits.
You reached a key decision on the regulation of some types of CRISPR-Cas9 gene-edited plants. Before we get to that, could you start by explaining what led your team to start working on this issue?
It started when we received questions from two universities about whether they needed to apply for permission to undertake field trials with some plant lines modified using CRISPR/Cas9. The underlying question was whether these plants are included in the gene technology directive or not. According to the Swedish service obligation for authorities, the SBA had to deliver an answer, and thus had to interpret the directive on this point.
Image credit: INRA, Jean Weber. Used under license: CC BY 2.0.
Could you give a brief overview of Sweden’s analysis of the current EU regulations that led to your interpretation that some CRISPR-Cas9 gene-edited plants are not covered by this legislation?
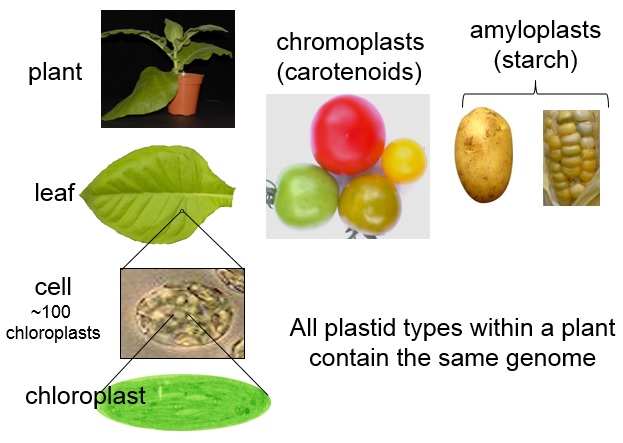
The following simplification describes our interpretation pretty well; if there is foreign DNA in the plants in question, they are regulated. If not, they are not regulated.
Our interpretation touches on issues such as what is a mutation and what is a hybrid nucleic acid. The first issue is currently under analysis in the European Court of Justice. Other ongoing initiatives in the EU may also change the interpretations we made in the future, as the directive is common for all member states in the EU.
CRISPR-Cas9 is a powerful tool that can result in plants with no trace of transgenic material, so it is impossible to tell whether a particular mutation is natural. How did this influence your interpretation?
We based our interpretation on the legal text. The fact that one cannot tell if a plant without foreign DNA is the progeny of a plant that carried foreign DNA or the result of natural mutation strengthened the position that foreign DNA in previous generations should not be an issue. It is the plant in question that should be the matter for analysis.

Image credit: Frost Museum. Used under license: CC BY 2.0.
Does your interpretation apply to all plants generated using CRISPR-Cas9, or a subset of them?
It applies to a subset of these gene-edited plants. CRISPR/Cas9 is a tool that can be used in many different ways. Plants carrying foreign DNA are still regulated, according to our interpretation.
What does your interpretation mean for researchers working on CRISPR-Cas9, or farmers who would like to grow gene-edited crops in Sweden?
It is important to note that, with this interpretation, we don’t remove the responsibility of Swedish users to assess whether or not their specific plants are included in the EU directive. We can only tell them how we interpret the directive and what we request from the users in Sweden. Eventually I think there will be EU-wide guidelines on this matter. I should add that our interpretation is also limited to the types of CRISPR-modified plants described in the letters from the two universities.

Will gene-edited crops be grown in Europe in the future? Image credit: Richard Beatson. Used under license: CC BY 2.0.
We are currently waiting for the EU to declare whether CRISPR-Cas9 gene-edited plants will be regulated in Europe. Have policymakers in other European countries been in contact with you regarding Sweden’s decision process?
Yes, there is a clear interest; for example, Finland handled a very similar case. Other European colleagues have also shown an interest.
What message would you like plant scientists to take away from this interview? If you could help them to better understand one aspect of policymaking, what would it be?
Our interpretation is just an interpretation and as such, it is limited and can change as a result of what happens; for example, what does not require permission today may do tomorrow. Bear this in mind when planning your research and if you are unsure, it is better to ask. Moreover, even if the SBA (or your country’s equivalent) can’t request any information about the cultivation of plants that are not regulated, it is good to keep us informed.
I think it is vital that legislation meets reality for any subject. It is therefore good that pioneers drive us to deal with difficult questions.