Author: Ansar Ali @Ansar_IPMB, Ph.D. Student at Institute of Plant and Microbial Biology @IPMBsinica, Academia Sinica, Taiwan in @KimmyHo_Lab.
Stomata are microscopic structures on the leaf epidermis that facilitate gas exchange between the plant and the environment. Thanks to these specialized cell types, plants are an essential component of the global carbon and water cycles. A 40% decrease in stomatal density was discovered in herbarium specimens collected over the last 200 years, most likely as a result of the rise in atmospheric carbon dioxide (CO2) concentrations since the beginning of the industrial revolution (Woodward, 1987). Rising CO2 concentrations could also be linked to the observed increase in forest water use efficiency (WUE, grain produced per unit of water used by the crop) due to stomatal partial closure (Keenan et al., 2013). Hence, stomata not only interlink plants and climate but also influence the global water cycle.
Introduction to “stomatal genes”
Stomatal distribution on the leaf surface in most plants obeys the one-cell-spacing rule, which states that a stomate is separated from another stomate by at least one pavement cell. This cell-to-cell communication in Arabidopsis is mediated by a signaling pathway initiated by EPIDERMAL PATTERNING FAMILY (EPF) peptide ligands (Hunt & Gray, 2009) and the ERECTA family of receptors and its co-receptor TOO MANY MOUTHS (TMM). TMM dysfunction causes contiguous stomatal clusters and an increase in the density of palisade mesophyll cells. Surprisingly, tmm mutants have higher WUE than wild-type plants, implying that WUE is controlled by interlayer coordination between the epidermis and the mesophyll (Dow et al., 2017). A recent study by Yang., et al, revealed that changes in the cuticle-cell wall continuum have an impact on stomatal development and cause stomatal clustering since the cuticle protects the aerial epidermis of all land plants from desiccation and other external environmental stresses. Their findings suggest that cutin plays an important role in epidermal sealing and that the altered composition of cutin and wax in the cuticle may affect water permeability. Although TMM has been extensively studied in Arabidopsis, its function in other plant species with clustered stomata is unknown.
Recent results on Begonia
In a recent study, Tsai et al. investigated the interdependence of stomatal morphology and WUE in two Asian Begonia species, B. formosana and B. hernandioides (Figure 1). B. hernandioides (with clustered stomata) and B. formosana (with solitary stomata) belong to different environments with the former suffering partial water shortage and the later experiencing no water deficit.
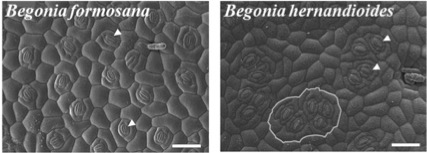
Figure 1. Stomatal morphologyB. formosana (with solitary stomata) and B. hernandioides (with clustered stomata). Arrowheads indicate guard cells. Scale bars, 100 μm
B. formosana is widely distributed on the forest floors of Taiwan, the Ryukyus Islands, and Japan while B. hernandioides is found in northern Luzon and the Philippines along the coastal limestone hills. The authors investigated the factors influencing the different gas exchange strategies used by these two Begonia species by looking at intrinsic WUE (WUEi), leaf characteristics, and stomatal responses. They also investigated the function of TMM in Begonia species with different stomatal types to see if TMM is involved in the formation of stomatal clustering in Begonia. They discovered that Begonia plants with clustered stomata have higher WUEi than those with solitary stomata. The multiseriate epidermis and a rapid and synchronous stomatal response under saturated light conditions (100-200 molm2s1) in B. hernandioides were responsible for this increase in WUEi. Furthermore, complementation of the Arabidopsis tmm mutant was more effective in inhibiting stomatal formation in Arabidopsis when the TMM locus from the solitary stomata Begonia species was used rather than TMM from the clustered stomata Begonia species.
Despite the fact that these two Begonia species have been grown in the same environment (greenhouse) for many years, the differences in their habitats and WUE suggest a link between environmental adaptation and genetic control of stomata development.
References
Woodward, F. I. (1987). Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature, 327(6123), 617– 618. https://doi.org/10.1038/327617a0
Keenan, T. F., Hollinger, D. Y., Bohrer, G., Dragoni, D., Munger, J. W., Schmid, H. P., & Richardson, A. D. (2013). Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature, 499(7458), 324– 327. https://doi.org/10.1038/nature12291
Hunt, L., & Gray, J. E. (2009). The signaling Peptide EPF2 Controls asymmetric cell divisions during stomatal development. Current Biology, 19(10), 864– 869. https://doi.org/10.1016/j.cub.2009.03.069
Peterson, K. M., Rychel, A. L., & Torii, K. U. (2010). Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. The Plant Cell, 22(2), 296– 306. https://doi.org/10.1105/tpc.109.072777
Dow, G. J., Berry, J. A., & Bergmann, D. C. (2017). Disruption of stomatal lineage signaling or transcriptional regulators has differential effects on mesophyll development, but maintains coordination of gas exchange. New Phytologist, 216(1), 69– 75. https://doi.org/10.1111/nph.14746
Tsai, M.Y., Kuan, C., Guo, Z.L., Yang, H.A., Chung, K.F., & Ho, C.M.K. (2022) Stomatal clustering in Begonia improves water use efficiency by modulating stomatal movement and leaf structure. Plant-Environment Interactions, 3(4), 141-154. https://doi.org/10.1002/pei3.10086
Yang, S.L., Tran, N., Tsai, M.Y., & Ho, C.M.K Misregulation of MYB16 expression causes stomatal cluster formation by disrupting polarity during asymmetric cell divisions, The Plant Cell, 34(1),455–476, https://doi.org/10.1093/plcell/koab260
Image: Marjon Besteman / Pixabay







