In plants, disease resistance proteins serve as major immune receptors that sense pathogens and pests and trigger robust defense responses. Scientists previously found that one such disease resistance protein, ZAR1, is transformed into a highly ordered protein complex called a resistosome upon detection of invading pathogens, providing the first clue as to how plant disease resistance proteins work. Precisely how a resistosome activates plant defenses, however, has been unclear.
A joint team led by Profs. ZHOU Jianmin, CHEN Yuhang and HE Kangmin at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences and Prof. CHAI Jijie at Tsinghua University recently employed state-of-the-art electrophysiology and single-molecule imaging to investigate the molecular mechanism by which the ZAR1 resistosome activates plant immunity.
By using Xenopus oocyte- and planar lipid bilayer-based electrophysiology studies, the researchers first showed that the ZAR1 resistosome is a cation-selective, calcium-permeable ion channel. They then applied single-molecule imaging to show that the activated ZAR1 resistosome forms pentameric oligomers in the plasma membrane of the plant cell, confirming previous structural data.
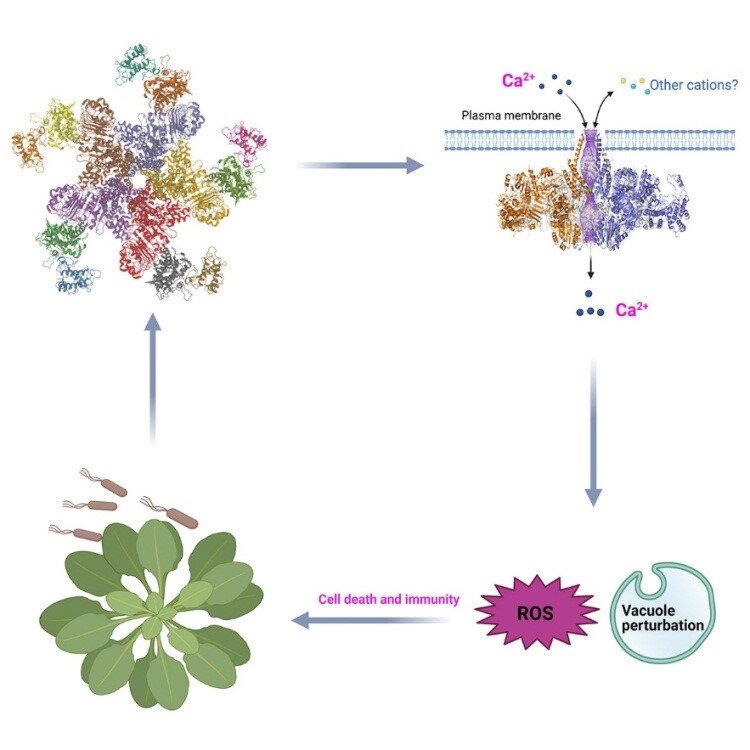
The ZAR1 resistosome acts as an ion channel in the plasma membrane to trigger Ca2+ ion flux and immune responses. (adopted from Bi et al., Cell)
The formation of ZAR1 resistosome in the plant cell triggers sustained calcium ion influx and subsequent immune signaling events leading to cell death, and these processes are all dependent on the activity of the ion channel.
Together, these results support the conclusion that the calcium signal triggered by the ZAR1 channel initiates immune activation, thus providing crucial insights into the working of plant immune systems.
Disease resistance proteins are the largest family of plant immune receptors and are of major agricultural importance in protecting crop plants from assault by diverse pathogens and pests including viruses, bacteria, fungi, oomycetes, nematodes, insects, and parasitic weeds.
The findings of this study shed light on the precise biochemical function of many disease resistance proteins, and suggest new methods for controlling diseases and pest damage in crop plants.
This work “presents important findings that will change our view of ETI-triggered cell death,” said a reviewer from Cell. “The use of TIRF to visualize and monitor in real-time membrane-associated resistosomes is very exciting and many researchers will strive to emulate this method.”
Read the paper: Cell
Article source: Chinese Academy of Sciences
Author: ZHANG Nannan
Image credit: CCO







