A new study published in the journal Nature shows that the same phenomenon that occurs when we try to mix oil and water – phase separation – plays an important role in the immune system of plants.
In a groundbreaking study, researchers led by Jijie Chai at Westlake University, China, Jane Parker and Paul Schulze-Lefert at the Max Planck Institute for Plant Breeding Research in Cologne, Germany, have demonstrated that an important class of immune proteins must condense into droplets to become activated and protect plants against infections.
The scientists set out to discover how a class of plant immune receptors called Toll/Interleukin-1 receptor (TIR) nucleotide-binding leucine-rich repeat (NLR) receptors (TNLs) are activated. TNLs are defined by the presence of a TIR domain, an ancient protein module that has roles in immunity from bacteria to mammals, but for activation they typically rely on additional domains that recognize molecules derived from invading pathogens. The TIR domain acts as an enzyme and consumes substrates in host cells that are important for energy metabolism. Activated TNLs often go on to trigger cell death at the site of attempted infection as a protective response to safeguard the entire plant. In addition to the typical TNLs, plants also have TNLs that lack those elements that recognize invaders, whilst retaining the ability to promote cell death and protect plants against infection. Although it was known that these truncated TNLs, known as TIR domain proteins, like to interact with each other when activated by their substrates and that, once activated, they function as enzymes to propagate the immune response, how precisely this happens has remained unknown. The researchers have now found that, as the concentrations of TIR domain proteins rise inside plant cells, they separate out like drops of oil in water – a typical feature of proteins that interact with each other – that trigger the immune-related cell death response. Further, these structures are not static with TIR domain proteins constantly moving in and out of the droplets.
Through the concentration and organization of enzyme assemblies, phase separation is an effective way to boost enzyme activity. Forming such condensates is common for animal immune proteins and, although it was known that the phenomenon was important for plant immune responses, it was unclear why.
Beyond the fundamental insight into how plant immune molecules are activated, the discovery of the phase separation-based mechanism for activation of TIR domain proteins may have implications for scientists’ understanding of how the different branches of plant immunity talk to each other. Co-corresponding author Schulze-Lefert is fascinated by the diversity of different assemblies of TIR domains in large protein complexes.
“It is simply amazing how evolution has repeatedly found ways to endow the ancient TIR protein structure in plants, animals and bacteria with new biochemical qualities that are ultimately important for the robustness of an immune system.”
Read the paper: Nature
Article source: Max Planck Institute for Plant Breeding Research, Köln
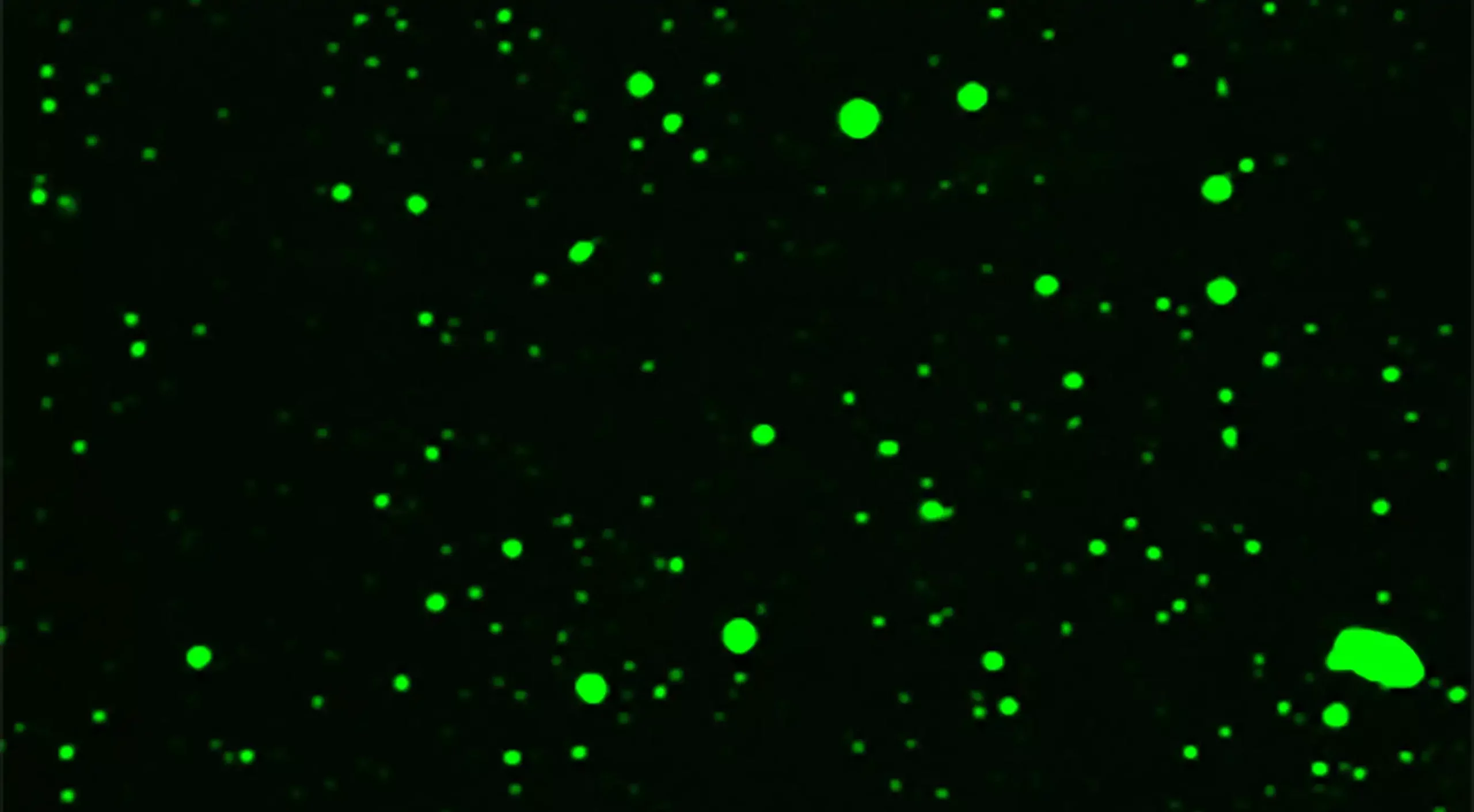
Image: Substrate-induced droplet formation of a plant TIR domain in vitro. The Arabidopsis TIR domain protein RPP1 was fused with the fluorescent protein GFP so that the rapid droplet formation of the RPP1-GFP fusion protein can be visualized by fluorescence microscopy after addition of the substrates NAD+ or ATP in a test tube. The droplets are highly dynamic structures. Credit: Max Planck Institute for Plant Breeding Research, Köln