Planting Parasites: Unveiling Common Molecular Mechanisms of Parasitism and Grafting
β-1,4-glucanase, a cell wall degrading enzyme, is integral for plant parasitism and cross-species grafting in the plant family Orobanchaceae.
Using the model Orobanchaceae parasitic plant Phtheirospermum japonicum, scientists from Nagoya University and other research institutes from Japan have discerned the molecular mechanisms underlying plant parasitism and cross-species grafting, pinpointing enzyme β-1,4-glucanase (GH9B3) as an important contributor to both phenomena. Targeting this enzyme may help control plant parasitism in crops. Also, this mechanism can be exploited for novel cross-species grafting techniques to realize the goal of sustainable agricultural technologies.
Plant parasitism is a phenomenon by which the parasite plant latches onto and absorbs water and nutrients from a second host plant, with the help of a specialized organ called the “haustorium.” Once the haustorium forms, specific enzymes then help in forming a connection between the tissues of the parasite and host plants, known as a “xylem bridge,” which facilitates the transport of water and nutrients from the host to the parasite.
A similar mechanism is involved in the process of artificial stem grafting, during which, the cell walls of the two different plant tissues at the graft junction become thinner and compressed, a phenomenon made possible by specific cell wall modifying enzymes. Cell wall modification has also been implicated to play a role in parasitism in different lineages of parasitic plants.
Therefore, the research team, led by Dr Ken-ichi Kurotani of Nagoya University, hypothesized that similar genes and enzymes should be involved in the process of parasitism and cross-species grafting. “To investigate molecular events involved in cell-cell adhesion between P. japonicum and the host plant, we analyzed the transcriptome for P. japonicum–Arabidopsis parasitism and P. japonicum–Arabidopsis grafting,” reports Dr Kurotani. When a gene in a cell is activated, it produces an RNA “transcript” that is then translated into an active protein, which is then used by the cell to perform various activities. A “transcriptome” is the complete set of RNA transcripts that the genome of an organism produces under various diverse conditions. The findings of their experiments are published in Nature’s Communications Biology.
Comparison of the parasitism and graft transcriptomes revealed that genes associated with wound healing, cell division, DNA replication, and RNA synthesis were highly upregulated during both events, indicating active cell proliferation at both the haustorium and graft interface.
“What’s more, we found an overlap between the transcriptome data from this study and that from grafting between Nicotiana and Arabidopsis, another angiosperm,” reports Dr Michitaka Notaguchi, the co-corresponding author of the study. Glycosyl hydrolases are enzymes that specifically target the breakdown of cellulose, the primary component of plant cell walls. A β-1,4-glucanase identified in P. japonicum belongs to the glycosyl hydrolase 9B3 (GH9B3) family; an enzyme from the same family was recognized to be crucial for cell-cell adhesion in Nicotiana by Dr Notaguchi‘s group.
Further experiments showed that GH9B3-silenced P. japonicum could form the haustorium with Arabidopsis but could not form a functional xylem bridge, meaning that the P. japonicum β-1,4-glucanase is integral for the plant’s parasitic activity. Further, high GH9B3 RNA transcript levels were observed during artificial grafting experiments, thereby proving that the enzyme plays an integral role in both parasitism and grafting mechanisms.
The transcriptome data generated in this study can be used to unearth additional genes and enzymes involved in plant parasitism. Additionally, further research along these directions will help scientists develop specific molecular approaches to arrive at sustainable cross-species grafting alternatives.
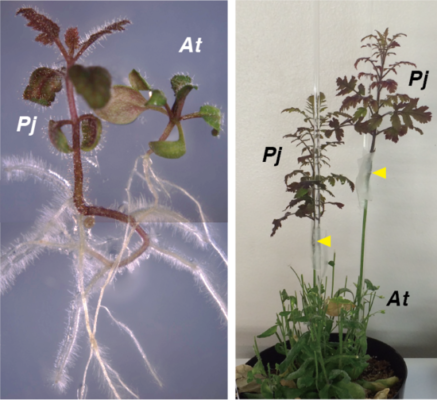
(Left) Parasitism between the roots of P. japonicum and Arabidopsis
(Right) Grafting of P. japonicum with Arabidopsis
Yellow arrow heads indicate the grafted points. Credit: Michitaka Notaguchi
Read the paper: Communications Biology
Article source: Nagoya University
Author: Michitaka Notaguchi
Image credit: Wikimedia






