Optogenetics can be used to activate and study cells in a targeted manner using light. Scientists at the University of Würzburg have now succeeded in transferring this technique to plants.
It is almost ten years since the scientific journal Science called optogenetics the “breakthrough of the decade”. Put simply, the technique makes it possible to control the electrical activity of cells with pulses of light. With its help, scientists can gain new insights into the functioning of nerve cells, for example, and thus better understand neurological and psychiatric diseases such as depression and schizophrenia.
Established procedure on animal cells
In research on animal cells, optogenetics is now an established technique used in many fields. The picture is different in plant research: transferring the principle to plant cells and applying it widely has not been possible until now.
However, this has now changed: Scientists at the Julius Maximilians University of Würzburg (JMU) have succeeded in applying optogenetic methods in tobacco plants. They present the results of their work in the current issue of the journal Nature Plants. “In particular, Dr. Kai Konrad from Prof. Hedrich’s group (Botany I) and Dr. Shiqiang Gao from my group were mainly responsible for the success of this project” explains Professor Georg Nagel, co-founder of optogenetics. In addition to the Department of Neurophysiology in the Institute of Physiology, three chairs of the Julius-von-Sachs Institute were involved in the collaboration: Botany I, Botany II and Pharmaceutical Biology.
Light switch for cell activity
“Optogenetics is the manipulation of cells or living organisms by light after a ‘light sensor’ has been introduced into them using genetic engineering methods. In particular, the light-controlled cation channel channelrhodopsin-2 has helped optogenetics achieve a breakthrough,” says Nagel, describing the method he co-developed. With the help of channelrhodopsin, the activity of cells can be switched on and off as if with a light switch.
In plant cells, however, this has so far only worked to a limited extent. There are two main reasons for this: “It is difficult to genetically modify plants so that they functionally produce rhodopsins. In addition, they lack a crucial cofactor without which rhodopsins cannot function: all-trans retinal, also known as vitamin A,” explains Dr. Gao.
Green light for plant cells
Prof. Nagel, Dr. Gao, Dr. Konrad, and colleagues have now been able to solve both problems. They have succeeded in producing vitamin A in tobacco plants by means of an introduced enzyme from a marine bacterium, thus enabling improved incorporation of rhodopsin into the cell membrane. This allows, for the first time, non-invasive manipulation of intact plants or selected cells by light via the so-called anion channel rhodopsin GtACR1.
In an earlier approach, plant physiologists from Botany I had artificially added the much-needed cofactor vitamin A to cells to allow a light-gated cation channel to become active in plant cells (Reyer et al., 2020, PNAS). Using the genetic trick now presented, Prof. Nagel and colleagues have generated plants that produce a special enzyme in addition to a rhodopsin, called dioxygenase. These plants are then able to produce vitamin A – which is normally not present in plants – from provitamin A which is abundant in the plant chloroplast. The combination of vitamin A production and optimization of rhodopsins for plant application ultimately led the researchers led by Prof. Nagel, Dr. Konrad and Dr. Gao to success.
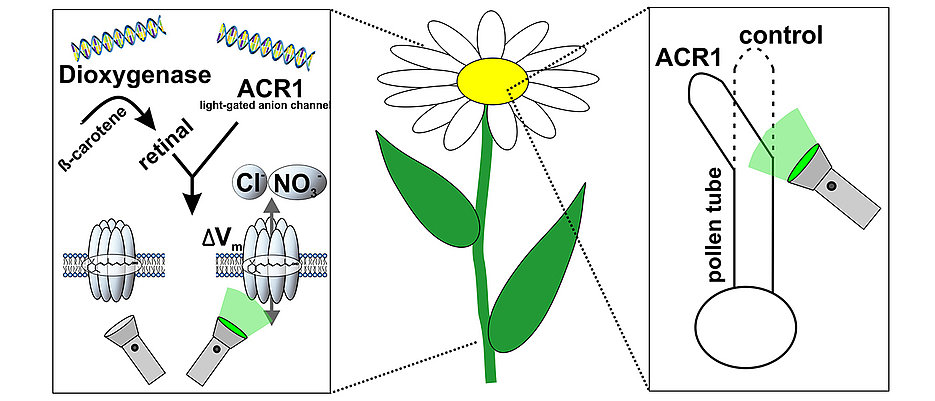
CAPTION. With two additional genes for the enzyme dioxygenase and the light-controlled anion channel ACR1, the tobacco plant can channel salt ions across the cell membrane when exposed to green light. The success can be seen in the experiment: While pollen tubes normally grow in the direction of the egg cell for fertilization, in genetically modified cells they change the direction of growth depending on the exposure to light. Credit: Dr. Kai Konrad.
New approach for plant research
“If you irradiate these cells with green light, the permeability of the cell membrane for negatively charged particles increases sharply, and the membrane potential changes significantly,” explains Dr. Konrad. In this way, he says, it is possible to specifically manipulate the growth of pollen tubes and the development of leaves, for example, and thus to study the molecular mechanisms of plant growth processes in detail. The Würzburg researchers are confident that this novel optogenetic approach to plant research will greatly facilitate the analysis of previously misunderstood signaling pathways in the future.
Read the paper: Nature Plants
Article source: University of Würzburg
Author: Gunnar Bartsch
Image credit: The Polish / Wikimedia








