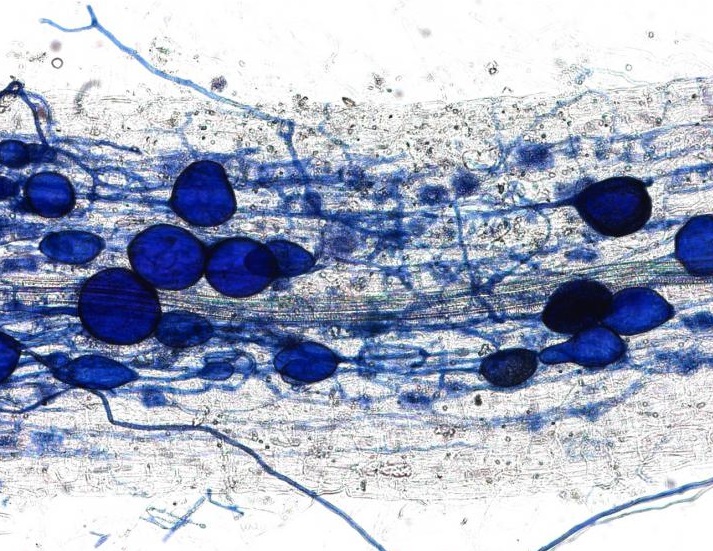
This week’s post comes to us from Crystal Chan, project manager of the Application of Genomic Innovation in the Lentil Economy project led by Dr. Kirstin Bett at the Department of Plant Sciences, University of Saskatchewan.
Could you begin with a brief introduction to your research?
Our research focuses on the smart use of diverse genetic materials and wild relatives in the lentil (Lens culinaris) breeding program.
Canada has become the world’s largest producer and exporter of lentils in recent years. Lentils are an introduced species to the northern hemisphere and, until recently, our breeding program at the University of Saskatchewan involved just a handful of germplasms adapted to our climatic condition. With dedicated breeding efforts we have achieved noteworthy genetic gains in the past decade, but we are missing out on the vast genetic diversity available within the Lens genus. This is a major dilemma faced by all plant breeders: do we want consistency (sacrificing genetic diversity and reducing genetic gains over time) or diversity (sacrificing some important fixed traits and spending lots of time and resources in “backcrossing/rescue efforts”)?
In our current research, we use genomic tools to understand the genetic variability found in different lentil genotypes and the basis of what makes lentils grow well in different global environments (North America vs. Mediterranean countries vs. South Asian countries). We will then develop molecular breeding tools that breeders can use to improve the diversity and productivity of Canadian lentils while maintaining their adaptation to the northern temperate climate.
What first led you to this research topic?
Dr. Albert (Bert) Vandenberg, professor and lentil breeder at the University of Saskatchewan, noticed one of the wild lentil species was resistant to several diseases that devastate the cultivated lentil. After years of dedicated breeding effort, he was able to transfer the resistance traits to the cultivated lentil, but it took a lot of time and resources. We began looking into other beneficial traits and became fascinated with the domestication and adaptation aspects of lentil – after all lentil is one of the oldest cultivated crops, domesticated by man around 11,000 BC! With the rapid advance in genomic technology, we can start to better understand the biology and develop tools to harness these valuable genetic resources.
You have been involved in the development of tools that assist researchers to build databases of genomics and genetics data. Could you tell us more about projects such as Tripal?
Over the past six years, Lacey Sanderson (bioinformaticist in our group) has developed a database for our pulse research program at the University (Knowpulse, http://knowpulse.usask.ca/portal/). The database is specifically designed to present data that is relevant to breeders, as our group has a strong focus on variety development for the Canadian pulse crop industry. Knowpulse houses genotypic information from past and on-going lentil genomics projects, and includes tools for looking up genotypes as well as comparing the current genome assembly (currently v1.2) and other sequenced legume genomes. The tools are being developed in Tripal, an open-source toolkit that provides an interface between the data and a Drupal web content management system, in collaboration with colleagues at Washington State University.
At the moment we are developing new functionalities that will allow us to store and present germplasm information as well as phenotypic data. We are also working with our colleagues at Washington State University (under the “Tripal Gateway Project” funded by the National Science Foundation) to enhance interconnectivity between Knowpulse and other legume databases, such as the Legume Information Service (LIS) and Soybase, to facilitate comparative genomic studies.
How challenging are pulse genomes to assemble? How closely related are the various crops?
We had the fortune to lead the lentil genome sequencing initiative thanks to the support from producer groups and governments across the globe. The lentil genome is really challenging to assemble! We see nice synteny between lentil and the model legume, medicago, however the lentil genome is much bigger. We see a significant increase in genome size between chickpea and beans versus lentil (and pea for that matter), yet we have evidence to show that genome duplication is not the cause of the size increase. There are a lot of very long repetitive elements sprinkled around the genome, which makes its sequencing and proper assembly very challenging. Not to mention understanding the role of these long repetitive elements in biological functions…
What insights into crop domestication have you gained from these genomes?
That’s what we are working on right now under the AGILE (“Application of Genomics to Innovation in the Lentil Economy”) project. Stay tuned!
Do you work with breeders to develop new cultivars? What sorts of traits are most important?
Breeding is at the core of our work – both Kirstin and Bert are breeders (Kirstin has an active dry bean breeding program when she’s not busy with genomic research). All our research aims to feed information to the breeders so that they can make better crossing and selection decisions. Our work in herbicide tolerance has led to the development and implementation of a molecular marker to screen for herbicide resistance. With that marker we save time (skipping a crossing cycle) and forego the herbicide spraying test for all of our early materials.
Disease resistance and drought tolerance are also important for the growers. Visual quality (seed shape, size, color) are very important too as our customers are very picky as to what sort of lentils they like to buy/eat.
What does the future of legume/lentil agriculture hold?
Lentils have been a staple food in many countries for centuries and have been gaining popularity in North America in recent years as people are looking for plant-based protein sources. Lentils are high in fibre, protein, and complex carbohydrates, while low in fat and calories, and have a low glycemic index. They are suitable for vegetarian/vegan, gluten-free, diabetic, and heart-smart diets. Lentils also provide essential micronutrients such as iron, zinc and folates. Lentils are widely recognized as nutrient-dense food that could serve as part of the solution to combat global food and nutritional insecurity.
In modern agriculture, adding lentil or other leguminous crops in the crop rotation helps improve soil structure, soil quality, and biotic diversity, as well as enhancing soil fertility through their ability to fix nitrogen. Because pulse crops require little to no nitrogen fertilizer, they use half of the non-renewable energy inputs of other crops, reducing greenhouse gas emissions.
2016 was marked by the United Nations as the International Year of Pulses, which was great as many people have become more aware of the benefits of pulse crops on the plate and in the field.
Follow us on twitter (@Wildlentils) for research updates!
All images are credited to Mr Derek Wright.