Many small regulatory elements, including miRNAs, miRNA binding sites, and cis-acting elements, comprise only 5~24 nucleotides and play important roles in regulating gene expression, transcription and translation, and protein structure, and thus are promising targets for gene function studies and crop improvement.
The CRISPR-Cas9 system has been widely applied in genome engineering. In this system, a sgRNA-guided Cas9 nuclease generates chromosomal double-strand breaks (DSBs), which are mainly repaired by nonhomologous end joining (NHEJ), resulting in frequent short insertions and deletions (indels) of 1~3 bp. However, the heterogeneity of these small indels makes it technically challenging to disrupt these regulatory DNAs. Thus, the development of a precise, predictable multi-nucleotide deletion system is of great significance to gene function analysis and application of these regulatory DNAs.
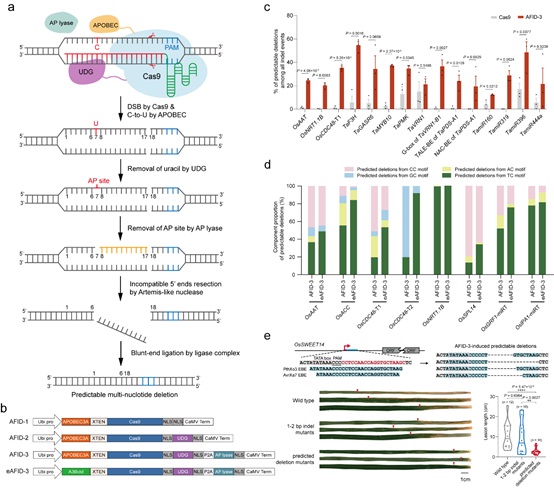
A research team led by Prof. GAO Caixia from the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences (CAS) has been focusing on developing novel technologies to achieve efficient and specific genome engineering. Based on the cytidine deamination and base excision repair (BER) mechanism, the researchers developed a series of APOBEC-Cas9 fusion-induced deletion systems (AFIDs) that combine Cas9 with human APOBEC3A (A3A), uracil DNA-glucosidase (UDG) and AP lyase, and successfully induced novel precise, predictable multi-nucleotide deletions in rice and wheat genomes.
“AFID-3 produced a variety of predictable deletions extending from the 5′-deaminated Cs to the Cas9 cleavage sites, with the average predicted proportions over 30%,” said Prof. GAO.
The researchers further screened the deamination activity of different cytosine deaminases in rice protoplasts, and found that the truncated APOBEC3B (A3Bctd) displayed not only a higher base-editing efficiency but also a narrower window than other deaminases.
They therefore replaced A3A in AFID-3 with A3Bctd, generating eAFID-3. The latter produces more uniform deletions from the preferred TC motifs to double-strand breaks, 1.52-fold higher than AFID-3.
Moreover, the researchers used the AFID system to target the effector-binding element of OsSWEET14 in rice, and found that the predictable deletion mutants conferred enhanced resistance to rice bacterial blight.
AFID systems are superior to other current tools for generating predictable multi-nucleotide targeted deletions within the protospacer, and thus promise to provide robust deletion tools for basic research and genetic improvement.
The team’s scientific paper, entitled “Precise, predictable multi-nucleotide deletions in rice and wheat using APOBEC–Cas9,” was published in Nature Biotechnology.
This research was supported by grants from the Strategic Priority Research Program of CAS, the National Transgenic Science and Technology Program and the National Natural Science Foundation of China.
Read the paper: Nature Biotechnology
Article source: Chinese Academy of Sciences
Author: ZHANG Nannan
Image credit: Tongpradit Charoenphon / Pixabay







