Lawrence Livermore National Laboratory (LLNL) scientists have developed a custom microscope to image microbes in soil and plants at the micrometer scale.
Live imaging of microbes in soil would help scientists understand how soil microbial processes occur on the scale of micrometers, where microbial cells interact with minerals, organic matter, plant roots and other microorganisms. Because the soil environment is both heterogeneous and dynamic, these interactions may vary substantially within a small area and over short timescales.
Imaging biogeochemical interactions in complex microbial systems, such as those at the soil−root interface, is crucial to studies of climate, agriculture and environmental health but complicated by the three-dimensional (3D) collocation of materials with a wide range of optical properties.
Microaggregates (<250 μm) formed and composed from microbial decomposition of soil organic matter are inhabited by distinct microbial communities potentially leading to highly divergent metabolisms and functions within the volume of soil that is typically sampled for molecular, genomic or physiochemical analyses. In addition to microbial heterogeneity, microorganisms also respond rapidly to changes in subsurface temperature, moisture, nutrient availability, signaling molecules and other conditions.
Researchers have pursued a large range of imaging techniques in efforts to understand the spatial and temporal aspects of these processes, but the combined characteristics of soil and microbes, including physical properties and length scales, continue to make monitoring and characterizing microbe-plant-soil interactions over time a significant challenge.
In recent years, some of the most impressive advances in imaging in soil have been achieved with X-ray computed tomography and magnetic resonance imaging. These modes are notable because they are capable of imaging deep into soil, and therefore they can provide unprecedented insight into plant root architecture, soil structure and even water movement. They have even been used for live imaging.
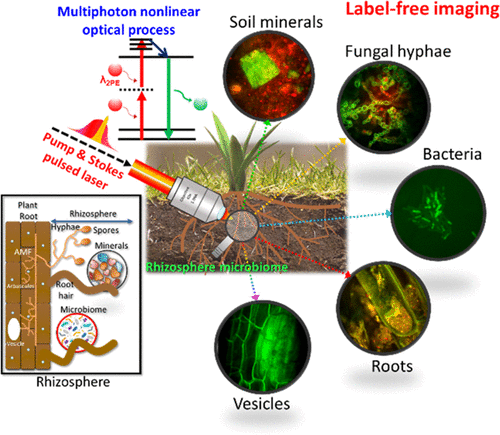
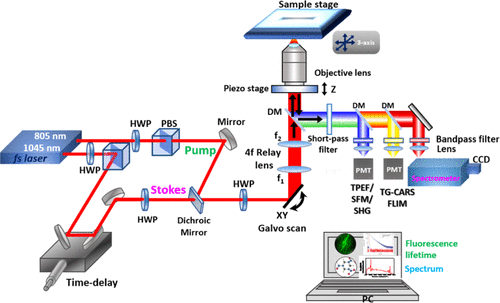
But these same methods are unable to image microbes such as individual hyphae and bacteria because of contrast or resolution limitations. The LLNL researchers turned to optical methods — imaging with light in the ultraviolet, visible and infrared spectrum — that allowed them to image microbes in soil and plants. “We wanted to image in the optical range because it is convenient, gentle and fast, but we knew that we needed to take a new approach to generating contrast to be able to image microbes in natural matrices,” said LLNL chemist Peter Weber, the project lead.
The team developed a label-free multiphoton nonlinear optics approach using multiple imaging modes to generate contrast and chemical information for soil microorganisms in roots and minerals.
“Multiphoton microscopy has multiple advantages over single-photon methods, like standard fluorescence imaging and Raman,” said LLNL physicist Janghyuk Lee, the lead author. “The No. 1 advantage is that it provides high signal with low risk of sample damage.”
The approach the LLNL team developed enables a strong signal for general microbe, plant and mineral imaging; high contrast, label-free chemical imaging that can target diagnostic biomolecules and minerals; very strong signals from specific minerals and some biomolecules; and higher information content, deeper penetration, less scattering, and less photodamage compared to confocal microscopy. The research appears in the journal Environmental Science and Technology.
Using this instrument, the team imaged symbiotic arbuscular mycorrhizal fungi structures within unstained plant roots in 3D to 60 μm depth. High-quality imaging was possible at up to 30 μm depth in a clay particle matrix and at 15 μm in a complex soil preparation.
“Our next step is to integrate adaptive optics into this system and attempt to image deeper,” said LLNL physicist Ted Laurence, a senior author on the study.
The technique allowed the researchers to identify previously unknown lipid droplets in the symbiotic fungus, Serendipita bescii. They also visualized unstained putative bacteria associated with the roots of Brachypodium distachyon in a soil microcosm.
“Our results show that this multimodal approach holds significant promise for rhizosphere and soil science research,” said LLNL physicist Sonny Ly, a senior author on the study. “We are particularly excited about the microscope’s potential for chemical imaging, such as identifying lipids in the fungus.”
Other LLNL scientists include Rachel Hestrin, Erin Nuccio, Jennifer Pett-Ridge, Keith Morrison, Christina Ramon and Ty Samo. The research is funded by the Bioimaging Science Program of the Department of Energy’s Office of Science.
Read the paper: Environmental Science and Technology
Article source: Lawrence Livermore National Laboratory
Author: Anne M Stark
Image credit: Goran Horvat / Pixabay







