Scientists have revealed new plant cell walls can have significantly different mechanical properties compared to surrounding parental cell walls, enabling cells to change their local shape and influence the growth of plant organs.
This is the first time that scientists have related mechanics to cell wall “age” and was only made possible through a new method that follows the same cells over time and through successive rounds of division.
The Cambridge researchers were able to see new walls forming and then measure their mechanical properties. This pioneering work showed that new cell walls in some plants are 1.5 times stiffer than the surrounding parental cell walls—an unexpected and surprising finding.
The size and shape of plant organs like leaves and flowers is the result of complex interactions between genetics, signaling, mechanical feedback, and environmental cues. While we have made a lot of progress in understanding these processes, it is not always easy to connect what happens at the cellular scale with what happens at the organ scale.
Research undertaken on two distantly related plant species at the Sainsbury Laboratory Cambridge University (SLCU) provides new evidence suggesting local level cell division has an active role to play in controlling organ size. The interdisciplinary project was a collaboration between three SLCU research teams (Robinson Group, Schornack Group and Jönsson Group) and the SLCU Microscopy Facilities Team, bringing together expertise in experimental biomechanics, genetics, imaging and computational modeling.
Combining advanced live microscopy imaging of individual cells, advanced material characterization methods, and mathematical modeling, Sarah Robinson’s research group has revealed the process of cell division locally alters the mechanical properties of the growing tissue, which potentially impacts on the final shape and size of the plant organ. The findings were published in Proceedings of the National Academy of Sciences.
Compared to animal cells, plant cells are enclosed by a rigid box—the cell wall. Cell division involves the addition of new cell walls, which alter the mechanical stress in the cell, its geometry and the mechanical properties of the surrounding tissue.
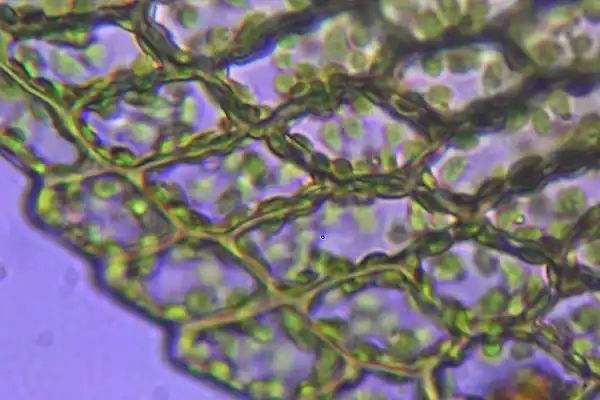
Scientists have been able to probe the mechanical properties of individual cell walls in the outer cell layer of a plant organ, but they did not know how old each wall is and could only guess if it had just divided. First author of the paper, former researcher in the Robinson Group and now a research fellow at Politecnico di Milano, Alessandra Bonfanti followed cells over time and could see new walls forming and therefore was able to relate mechanics to cell wall “age.”
Dr. Bonfanti developed a protocol that combines time-course imaging with atomic force microscopy measurements (AFM) to systematically map the age, growth and mechanical properties (stiffness) of individual cells walls and to follow the same cell walls through successive rounds of division.
“We have known for some time that the cell wall is a highly dynamic material. New material is added during cell division, while cell wall mechanical properties are modulated during growth to allow walls to undergo significant changes in shape and size without breakage,” Dr. Bonfanti said. “Yet, how the mechanical properties of new cell walls transiently change in space and time was still unknown until we developed a new protocol that allowed us to measure the mechanical properties of cell walls over time.”
“We used this protocol to address how the stiffness of newly formed cell walls varies at 24-hours and 48-hours up until its mature stage, and how this affects local cell shapes,” Dr. Bonfanti said. “To do so we made use of two systems: gemmae of the liverwort Marchantia polymorpha, and the early-stage first true leaf of Arabidopsis thaliana.”
The cells in the young tissues of the two plant species studied initially have a similar square-shaped geometry, which made them good models to compare.
“We first characterized the growth and cell division pattern in M. polymorpha gemmae, which was still unclear in the literature,” said Dr. Bonfanti. “Then, with the optomechanical measurements, using time course imaging combined with AFM measurements, we demonstrated that cell division in M. polymorpha gemmae results in the generation of a temporary stiffer and slower growing new wall. In contrast, this transient phenomenon is absent in A. thaliana leaves.”
In fact, the new cell walls in M. polymorpha became 1.5 times stiffer than the parental cell walls.
“We have shown that there are significant differences in the stiffness of new cells walls compared to parental walls and that these differences contribute to the cell’s geometry and growth,” Group Leader Dr. Robinson said. “This suggests cell division and its varying mechanical properties alters the rate of tissue expansion and could impact final organ size.”
Dr. Robinson explained the significance of the discovery: “We already knew that cell walls loosen and become softer when cells are growing as the walls must stretch so the cells can expand as they grow. But we didn’t know what would happen when a cell divides and what properties the resulting new cell wall would have. Would they be the same or different to the walls in the surrounding tissue and how this would this impact cell growth?”
“The fact that the new cell walls are much stiffer results in organ growth being restricted as it impedes the growth and influences the shape of component cells.”
“The M. polymorpha cells also change their geometry and develop a 120° junction angle quicker to form cell geometries closer to hexagonal shapes, which are thought to be the most efficient shapes in terms of forming a material to cover an area. The computational modeling done in this project by Euan Smithers and Ross Carter provided evidence that the presence of a stiff new wall accelerates the formation of these 120° angles.”
“It is important to know that the new cell wall can be different to the parental wall and this gives us new questions to explore—is that always the case, in what conditions, and why is this the case?”
Read the paper: PNAS
Article source: University of Cambridge
Image: The individual plants are usually composed of simple leaves that are generally only one cell thickwith no internal air spaces,They differ from vascular plants in lacking water-bearing xylem tracheids or vessels. Credit: Krishna satya 333/ Wikimedia