It’s the ancient story of plant evolution: photosynthetic algae moved to damp places on land, eventually evolving more complex architecture, and spreading across almost all terrestrial habitats. To cope with the drier conditions, plants developed roots to absorb water, and vascular tissue to transport it; a waxy cuticle coating their surfaces to prevent evaporation; and microscopic pores called stomata that open to allow carbon dioxide to diffuse in for photosynthesis but close to prevent excessive water loss.
How, then, does eelgrass (Zostera marina) fit in to this tale? It’s a monocot descended from the flowering plants, but it has turned its back on dry land and returned to the sea; a rare feat that only appears to have happened on three occasions. The recent sequencing of the eelgrass genome has revealed several interesting insights into the dramatic genetic changes that have allowed it to adapt to what lead author Professor Jeanine Olsen described as, “arguably the most extreme adaptation a terrestrial (and even a freshwater) species can undergo.”
Sayonara to stomata
If you live in the sea, conserving water isn’t your main concern. Eelgrass was known to lack stomata, but genetic comparisons to other species, including its freshwater relative Spirodela polyrhiza, revealed the first surprise of the study: eelgrass has lost not only its stomata but also the genes involved in their development and patterning. “The genes have just gone, so there’s no way back to land for seagrass,” said Olsen.
A difference in defense
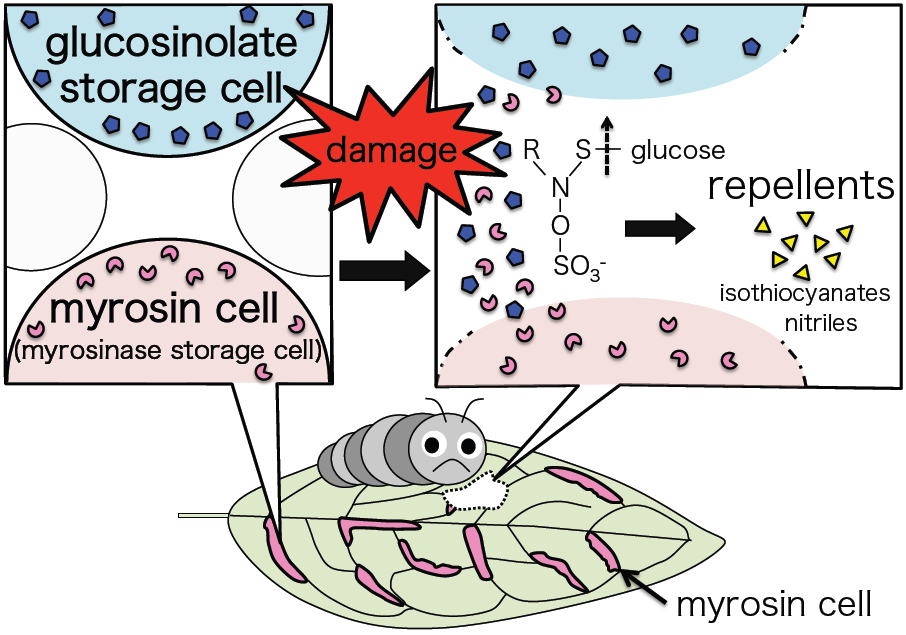
When angiosperms are attacked by herbivores or pathogens, their defense response typically involves the release of volatile secondary metabolites through their stomata. How can eelgrass release these compounds without stomata? The answer is: it doesn’t. The genome study found that eelgrass is missing crucial genes involved in making ethylene (an important hormone release in times of stress), as well as those responsible for producing non-metabolic terpenoids, which act to repel pests.
Selective pressures of the marine environment differ greatly from those of terrestrial habitats, so different pathways may be involved. Second, eelgrass has a wide repertoire of pathogen resistance genes, which suggests that it is exposed to a very different set of pathogens that may not respond to typical immune responses. Third, volatile secondary metabolites are often involved in attracting pollinators; this is not believed to be necessary in eelgrass, where submarine pollination occurs using the water itself.

Zostera marina – National Museum of Nature and Science, Tokyo. Public domain, CC0 1.0, via WikiMedia Commons.
Changing the cell wall
Eelgrass is subject to extremely salty conditions, and it’s had to adapt to osmotic stress. Unlike typical plant cell walls, eelgrass has engineered its cell wall matrix to retain water in the cell wall, even during low tide. This involves depositing sulfated polysaccharides and low methylated pectins in the cell wall matrix, but until its genome was sequenced no-one knew exactly how. It turns out that eelgrass has rearranged its metabolic pathways: “They have re-engineered themselves,” Olsen explains.
Living with a lack of light
Some species of Zostera can grow in water 50m deep, where light levels are reduced and shifted into a narrow wavelength range; ultraviolet (UV), red and far-red light have particularly low penetration after the first 1–2m of seawater. In a classic eelgrass ‘use it or lose it’ response, it has lost the UVR8 gene, which is responsible for sensing and responding to UV damage, as well as the phytochromes associated with red and far-red receptors. It does, however, retain the photosynthetic machinery, including photosystems I and II.
Unravelling angiosperm evolution
The recent eelgrass publication has revealed how this plant has either lost or adapted typical angiosperm traits to suit its needs, by ditching its stomata, volatile secondary metabolites and certain light sensing genes, or by altering the structure and function of the cell wall. It also developed adaptations that enable gas exchange, help pollen stick to submerged stigmas, and promote nutrient uptake.
Could these adaptations be useful in crop breeding? While a lack of defense compounds would probably be a step backwards, it would be extremely useful to understand how eelgrass copes with biotic stresses without them. Removing light receptors would also be problematic, but could eelgrass help us to develop crops that can grow in shaded conditions, perhaps in intercropping systems? What can we learn from eelgrass’ nutrient uptake and salt-tolerant adaptations?
Now that we have seen some of the secrets of eelgrass, how can we best make use of them?
Read the paper: The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea (Open Access)
Read the editorial: Genomics: From sea to sea (paywall)
Read the press release: Genome of the flowering plant that returned to the sea